What is it?
- The nitrogen cycle is a biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates through the atmosphere, and the terrestrial, and marine ecosystems.
- The conversion of nitrogen can be carried out through both biological and physical processes.
Stages
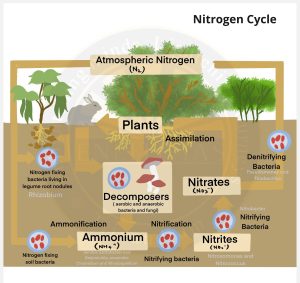
- Nitrogen Fixation
- Nitrogen is usable only after it is fixed.
- Nitrogen fixation is a process where bacteria convert N2 into ammonia, a form of nitrogen usable by plants.
- Non-Symbiotic Bacteria or Free-Living Bacteria– Azotobacter and Beijemickia (aerobic); Clostridium and Rhodospirillum (anaerobic).
- Symbiotic Bacteria– Rhizobium– live in association with leguminous root nodule plants, blue-green algae (Nostoc, Anabaena, Spirulina) are major sources of nitrogen fixation in oceans.
- The lightning and UV radiation also provide enough energy to convert nitrogen to nitrogen oxides.
- The industrial process like fertiliser factories also accomplish nitrogen fixation,
- Nitrification (Ammonia to Nitrates)
- Ammonium ions are directly taken up by some plants while most absorb nitrates obtained by oxidising ammonia and ammonium ions. Ammonium ions are first oxidised to nitrite by Nitrosomonas/ Nitrococcus bacteria.
- Nitrite is then oxidised to nitrate by Nitrobacter bacteria (chemoautotrophs).
- Plants absorb these nitrates and convert them into amino acids.
- Ammonification (Urea, Uric acid to Ammonia)
- Living organisms produce nitrogenous waste products like urea and uric acid.
- These waste products and dead remains of the organisms are converted back into inorganic ammonia and ammonium ions by bacteria by ammonification.
- Denitrification (Nitrate to Nitrogen)
- The process of reducing nitrate in the soil to nitrogen is called denitrification.
- Soil and oceans have denitrifying bacteria like Pseudomonas and Thiobacillus which convert nitrate/nitrites to elemental nitrogen.
- This nitrogen is released into the atmosphere completing the cycle.